COVID-19 Data & Vaccine Trends As The Country Moves Through Year 3 of the SARS-CoV-2 Virus Pandemic & Transitions to the Post Pandemic Era
COVID-19 Data Summary As of 9/24/2022 - Over two and a half years after the first seven (7) cases were identified in the U.S. in January of 2020.
- 97.9M positive COVID-19 cases have been reported, (30% to 35% of the total U.S. population - breakthrough cases not reported) from 1.2B reported COVID-19 tests, a 8.8% positive rate.
- 1.08M deaths have been reported, a 1.2% death rate versus a 98.8% recovery rate.
- 75.2% of all the deaths reported are from the age group 65 and older from only 11.6% of all the reported positive cases.
- 52.6% of all the positive cases reported are from the age group 18 to 49 years old and resulting in only 6.6% of all the reported deaths.
- 10.0% of all the positive cases reported to date are from the age group less than 12 years of age and until November 2, 2021 this age group was not yet eligible for the vaccine, resulting in 783 deaths or .096% of all reported deaths.
- 53.1% of the positive cases reported are female (50.75% of the U.S. population) vs. 45.4% of the reported deaths.
- 46.9% of the positive cases reported are male (49.2% of the U.S. population) vs. 54.6% of the reported deaths.
COVID-19 Vaccine Summary As of 9/20/2022 - Over two years after the first seven (7) cases were identified in the U.S. and nineteen (19) months after Operation Warp Speed was officially announced on 5/15/2020.
- 2 vaccines were authorized in December under the FDA Emergency Use Authorization authority: Pfizer-BioNTech on 12/11/2020 for use in individuals 16 years of age or older; Moderna on 12/18/2020 for use in individuals 18 years of age or older.
- A third vaccine from Johnson & Johnson, received FDA Emergency Use Authorization on February 27, 2021 and started shipping the first week in March. The Johnson & Johnson vaccine is a single dose vaccine vs the Pfizer and Moderna vaccines which are two dose vaccines.
- The Pfizer-BioNTech vaccine, on May 10, 2021 received expanded FDA Emergency Use Authorization age criteria to lower the minimum age for administration from the age of 16 to the age 12 and on November 2, 2021 the minimum age was lowered again to the age of 5.
- The age criteria expansions increased the potential U.S. population that could be vaccinated to 313M people or to 94.1% of the U.S. population.
- The Pfizer-BioNTech booster vaccine was authorized on 9/22/2021 and the Moderna booster on 10/20/2021 for use in individuals 65 years of age or older and individuals 18 through 64 years of age at high risk.
- 843.7M doses have been delivered.
- 616.2M total doses have been administered.
- 263.8M people ≥ 5 Years of age have received at least 1 dose, 83.2% of the 5 and older population.
- 225.0M people ≥ 5 Years of age are fully vaccinated, 70.3% of the 5 and older population.
- 233.4M people ≥ 18 Years of age have received at least 1 dose, 89.3% of the 18 and older population.
- 200.2M people ≥ 18 Years of age are fully vaccinated, 77.5% of the 18 and older population.
- 57.6M people ≥ 65 Years of age have received at least 1 dose, 97.5% of the 65 and older population.
- 50.6M people ≥ 65 Years of age are fully vaccinated, 92.3% of the 65 and older population.
- 109.6M people have received an additional booster dose since 9/22/2021.
- 49.5% of the people ≥ 12 Years of age that are fully vaccinated have also received a booster shot.
January 2020 COVID-19 Initial Timeline of Events
- 1/21/2020 the first case of COVID-19 in the U.S. was recorded.
- 1/30/2020 the CDC had confirmed the first case of person to person transmission in the U.S.
- 1/31/2020 seven cases in three states (Washington, Illinois and California) had been recorded with no deaths.
- 1/31/2020 HHS declared Coronavirus a Public Health Emergency in the U.S.
- 1/31/2020 the U.S. Centers for Disease Control and Prevention (CDC) issued a federal quarantine for 14 days affecting the 195 American evacuees from Wuhan, China.
- President Trump signed an order on Jan. 31 for the U.S. to deny entry to foreign nationals who traveled to China within the preceding two weeks, aside from the immediate family of U.S. citizens.
- 1/31/2020 Delta, American and United announced they would temporarily suspend all of their mainland China flights in response to the coronavirus outbreak.
WHG COVID-19 Data Tracking and Trends
The charts below represent a two year analysis by WHG of The United States COVID-19 Data and Trends.
- The Primary sources of the publicly available data is from Worldometer, an independent reference website, link listed below and the CDC. WHG tracks the data daily and calculates the trends and charts in a format to help our family, friends and clients stay informed.
- Worldometer's COVID-19 data is trusted and used by dozens of news outlets including Johns Hopkins CSSE and The New York Times.
- Source https://www.worldometers.info/coronavirus/country/us/
- The positive cases by age and the deaths by age data are direct downloads from the CDC website as of 1-5-2022.
- Please note that not all state and local public health agencies and/or private testing labs track or report age data (age data represents ~76% of all reported positive cases). https://covid.cdc.gov/covid-data-tracker/#demographics
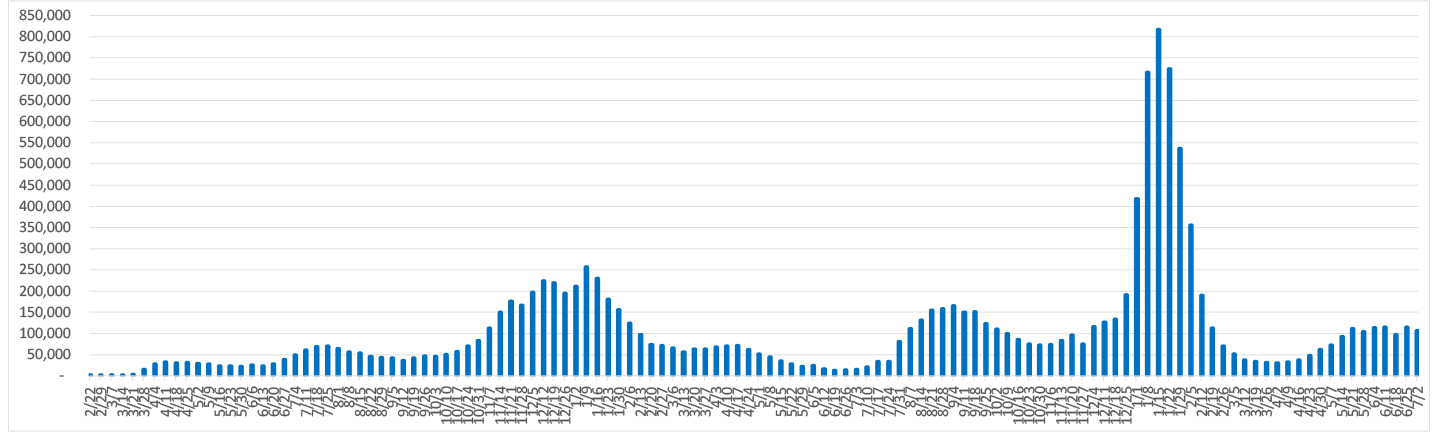
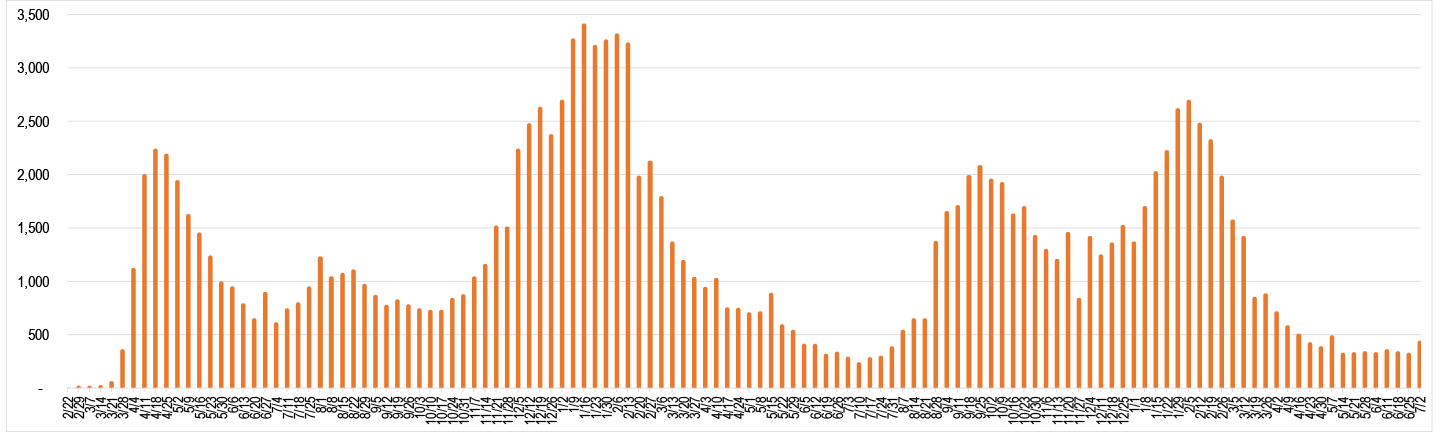
Worldometer Data and Trends as of 9/24/2021
Avg. # of New Positive Cases Per Day by Week
Avg. # of New Deaths Per Day by Week
|
|
|
Stay up to date on the latest news regarding COVID-19
- We all want to know how to keep ourselves and our families safe and healthy.
- COVID-19 Resources can provide helpful information in these challenging pandemic times.
- The following COVID-19 resources are provided so that you can access what’s important to you and your family. Testing, diagnosis, treatment and vaccinations for COVID-19 are all rapidly evolving.
Federal Government Websites
https://www.coronavirus.gov
https://www.cdc.gov
https://www.nih.gov
https://www.hhs.gov
https://www.fda.gov
https://www.cms.gov
https://www.coronavirus.gov
https://www.cdc.gov
https://www.nih.gov
https://www.hhs.gov
https://www.fda.gov
https://www.cms.gov
Data and Trending Informational Websites
US Data
https://www.worldometers.info/coronavirus/country/us/
World Data
https://www.worldometers.info/coronavirus/
CDC Data Tracking and Demographics
https://covid.cdc.gov/covid-data-tracker/#demographics
Track States’ Responses to Coronavirus – Provided by GovPredict
Testing - 2022 and Beyond
As the country moves forward reopening with the delta and omicron variant surges, one year after the launch of the first vaccines, 204.7M people have been fully vaccinated representing 65.6% of the age 5 and older population.
Even though the pace of vaccinations has slowed to 800K per day and the penetration rate of fully vaccinated people could soon approach 70% of the 5 and older population, COVID-19 testing will still be required and will continue to be part the public and private sector guidelines.
Currently 2.9M COVID-19 tests are being performed per day.
WHG would like to provide the information to help explain the differences between the two kinds of COVID-19 tests available today.
Even though the pace of vaccinations has slowed to 800K per day and the penetration rate of fully vaccinated people could soon approach 70% of the 5 and older population, COVID-19 testing will still be required and will continue to be part the public and private sector guidelines.
Currently 2.9M COVID-19 tests are being performed per day.
WHG would like to provide the information to help explain the differences between the two kinds of COVID-19 tests available today.
Two kinds of tests are available for COVID-19: : viral tests and antibody tests
A viral test tells you if you have a current infection.
A viral test tells you if you have a current infection.
- Authorized assays for viral testing include those that detect SARS-CoV-2 nucleic acid or antigen. Viral (nucleic acid or antigen) tests check samples from the respiratory system (such as nasal or oral swabs or saliva) to determine whether an infection with SARS-CoV-2, the virus that causes COVID-19, is present. Viral tests are recommended to diagnose acute infection of both symptomatic and asymptomatic individuals, to guide contact tracing, treatment options, and isolation requirements. Some tests are point-of-care tests, meaning results may be available at the testing site in less than an hour. Other tests must be sent to a laboratory, a process that may take at least 1-2 days.
An antibody test tells you if you had a previous infection
- FDA has not authorized using antibody tests to diagnose SARS-CoV-2 infection, and CDC does not currently recommend using antibody testing as the sole basis for diagnosis of acute infection. In certain situations, serologic assays may be used to support clinical assessment of persons who present late in their illnesses when used in conjunction with viral detection tests. In addition, if a person is suspected to have a post-infectious syndrome caused by SARS-CoV-2 infection (e.g., Multisystem Inflammatory Syndrome in Children; MIS-C), serologic assays may be used.
Serologic assays for SARS-CoV-2 infection, are an important tool for surveillance and epidemiologic studies, such as understanding the transmission dynamic of the virus in the general population. Unlike direct viral detection methods, such as nucleic acid amplification or antigen detection tests that can detect acutely infected persons, antibody tests help determine whether the individual being tested was previously infected—even if that person never showed symptoms.